Within the last year, global medical device manufacturer Exactech has recalled five joint replacement devices that show the potential to cause severe pain and injury to the patients who use them, sometimes even necessitating arduous revision surgery. Three of those devices are knee devices, more than 140,000 of which have been sold since 2004. If you have experienced pain or other complications from a recalled Exactech knee implant,
you may have a right to compensation. Here’s what you need to know.
Which Exactech Knee Devices Are Included in the Recall?
In August 2021, Exactech recalled knee replacements with a remaining shelf life of at least five years as of August 31, 2022. In February 2022, Exactech expanded on that recall, including three knee products regardless of the remaining shelf life. The recall involves the following knee replacement devices:
- Exactech Optetrak implants (60,926 sold since 2004)
- Exactech Optetrak Logic implants (60,518 sold since 2009)
- Exactech Truliant implants (24,727 sold since 2017)
Why Were These Knee Devices Recalled?
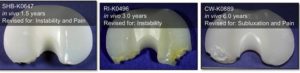
A defective polyethylene tibial insert prompted the recall. In its “Urgent Medical Device Correction” letter to healthcare professionals on February 7, 2022, Exactech acknowledged that “most of our inserts manufactured since 2004 were packaged in out-of-specification vacuum bags.”
[2] These vacuum bags are important because, according to Exactech's "Design Rationale," the inserts are placed in vacuum bags to prevent
oxidation, or exposure to oxygen. Oxidation resistance is an important safety consideration because the presence of excess oxygen can cause polyethylene to degrade, which can, in turn,
cause patients to suffer severe pain, bone loss, and possibly infection.
The Importance of Cross-Linking in Joint Replacements
To help prevent oxidation and degradation, manufacturers employ a process called “cross-linking.” Importantly, highly cross-linked polyethylene is shown to be resistant to oxidation and wear. Exactech’s tibial inserts have limited cross-linking because a high level of cross-linking was “not necessary.” Exactech explicitly promoted the wear resistance and longevity of its knee replacement devices.
[2] Although the recalled knee replacement implant devices have contained a defective tibial insert component since as early as 2004, Exactech took no action until their initial August 2021 recall.